
Separation Process Principles (3rd Edition) Edit editionThis problem has been solved:
Looking for the textbook?- CH1
- CH2
- CH3
- CH4
- CH5
- CH6
- CH7
- CH8
- CH9
- CH10
- CH11
- CH12
- CH13
- CH14
- CH15
- CH16
- CH17
- CH18
- CH19
- 1E
- 1SQ
- 2E
- 2SQ
- 3E
- 3SQ
- 4E
- 4SQ
- 5E
- 5SQ
- 6E
- 6SQ
- 7E
- 7SQ
- 8E
- 8SQ
- 9E
- 9SQ
- 10E
- 10SQ
- 11E
- 11SQ
- 12E
- 12SQ
- 13E
- 13SQ
- 14E
- 14SQ
- 15E
- 15SQ
- 16E
- 17E
- 18E
- 19E
- 20E
- 21E
- 22E
- 23E
- 24E
- 25E
- 26E
- 27E
- 28E
- 29E
- 30E
- 31E
- 32E
- 33E
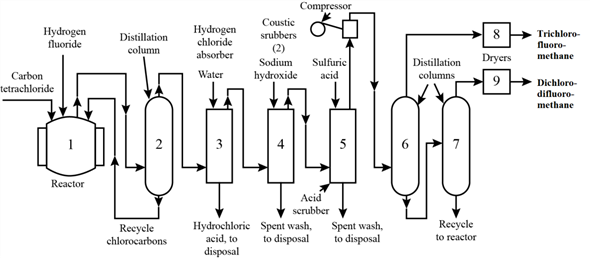
The process for the production of fluorocarbons from carbon tetrachloride and hydrogen fluoride comprises of a number of steps. The block-flow diagram for the process is given below:
Major reactions for the process are as follows:
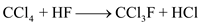
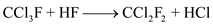
In the reactor (block-1), carbon tetrachloride (CCl4) reacts with hydrogen fluoride (HF) in the presence of catalyst antimony pentoxide (Sb2O5).
Main effluents from the reactor are hydrogen chloride (HCl), trichloro-fluoro-methane (CCl3F), dichloro-difluoro-methane (CCl2F2), unreacted CCl4, and small amounts of water and chlorine. Water comes from HF, which contains water as an impurity.
Effluents from the reactor (block-1), are sent to the distillation column (block-2) where CCl4 is removed as the bottom product and is recycled to the reactor. Distillate of the distillation column (block-2) is sent to the first absorber (block 3) for absorption of HCl by water. Aqueous HCl is obtained as the by-product.
Gas coming out of the first absorber (block-3) contains residual HCl, and chlorine (Cl2). Cl2 is absorbed by aqueous NaOH in the second absorber (block-4). Gas leaving this second absorber contains moisture, which is removed by absorption with Sulfuric acid (H2SO4) in third absorber (block-5).
Gas from third absorber (block-5) is sent to second distillation column (block-6) where CCl2F2 is obtained as a distillate, which is dried to remove the moisture. Bottom product of the second distillation column (block-6) is again sent to a distillation column (block-7) and CCl3F is obtained as distillate, which is also dried to get rid of the moisture.
Finally, the bottom product of the third distillation column (block-7) is recycled back to the reactor (block-1).
Corresponding textbook

Plus, we regularly update and improve textbook solutions based on student ratings and feedback, so you can be sure you're getting the latest information available.
The best part? As a Chegg Study subscriber, you can view available interactive solutions manuals for each of your classes for one low monthly price. Why buy extra books when you can get all the homework help you need in one place?



