
Organic Chemistry (3rd Edition) Edit editionThis problem has been solved:
Looking for the textbook?- CH1
- CH2
- CH3
- CH4
- CH5
- CH6
- CH7
- CH8
- CH9
- CH10
- CH11
- CH12
- CH13
- CH14
- CH15
- CH16
- CH17
- CH18
- CH19
- CH20
- CH21
- CH22
- CH23
- CH24
- CH25
- CH26
- CH27
- 1S
- 2S
- 3S
- 4S
- 5S
- 6S
- 7S
- 8S
- 9S
- 10S
- 11S
- 12S
- 13S
- 14S
- 15S
- 16S
- 17S
- 18S
- 19CCP
- 20CCP
- 21CCP
- 22S
- 23S
- 24CCP
- 25S
- 26S
- 27S
- 28S
- 29S
- 30S
- 31S
- 32S
- 33S
- 34PP
- 35PP
- 36PP
- 37PP
- 38PP
- 39PP
- 40PP
- 41PP
- 42PP
- 43PP
- 44PP
- 45PP
- 46PP
- 47PP
- 48PP
- 49PP
- 50PP
- 51PP
- 52PP
- 53PP
- 54PP
- 55PP
- 56PP
- 57PP
- 58PP
- 59PP
- 60PP
- 61PP
- 62PP
- 63IP
- 64IP
- 65IP
- 66IP
- 67IP
- 68IP
- 69IP
- 70CP
- 71CP
- 72CP
- 73CP
- 74CP
- 75CP
- 76CP
- 77CP
a. The first step to determine the constitution of the following compounds is by determining the amount of bonds each compound can form. In CH4O, carbon can form four bonds, hydrogen can form one bond, and oxygen can form two bonds.
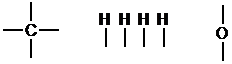
Atoms that can form the most bonds should be placed in the middle of the compound, while atoms that can only form one bond should be placed at the periphery. Here, oxygen and carbon can form the most bonds, so they are placed in the middle.
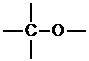
This structure leaves us with four places for bonds on the periphery, and we have four hydrogens left. This gives us the final constitution seen below.
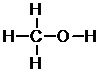
b. First, determine the amount of bonds each atom can form. Here, there will be four per carbon, one per hydrogen, and one per chlorine.
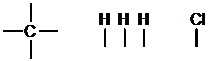
Atoms with the most bonds go in the center and atoms with only one bond go on the outside. Here, the central atom will be carbon. Since carbon forms four bonds and there are four atoms with one bond left, the final constitution is found by connecting these one-bond atoms to the central carbon.
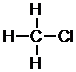
c. First, determine the number of bonds on each atom and then place the atoms that form the most bonds in the center. Here, the carbons form the most bonds and will be placed in the center.
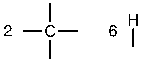
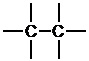
Next, place the atoms that form one bond around the periphery.
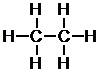
d. First, determine the number of bonds each atom can form. Nitrogen can form three bonds.
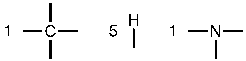
Then, place the atoms that form the most bonds in the center (C and N) and the one-bond atoms on the periphery.
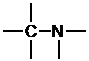
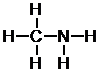
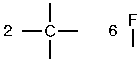
Next, place the atoms with the most bonds in the center, and the one-bond atoms on the outside.
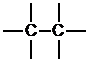
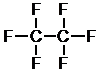
f. First, determine the number of bonds each atom is able to form – bromine is a halogen, so it can form one bond.
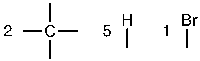
Next, place the atoms with the most bonds in the center, and the one-bond atoms on the edges.
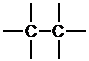
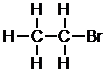
g. First, determine the number of bonds that each atom can form.
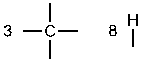
Next, place the atoms with the most bonds in the center, and the atoms that can only form one bond on the periphery.
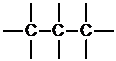
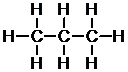
Corresponding textbook

Plus, we regularly update and improve textbook solutions based on student ratings and feedback, so you can be sure you're getting the latest information available.
The best part? As a Chegg Study subscriber, you can view available interactive solutions manuals for each of your classes for one low monthly price. Why buy extra books when you can get all the homework help you need in one place?



